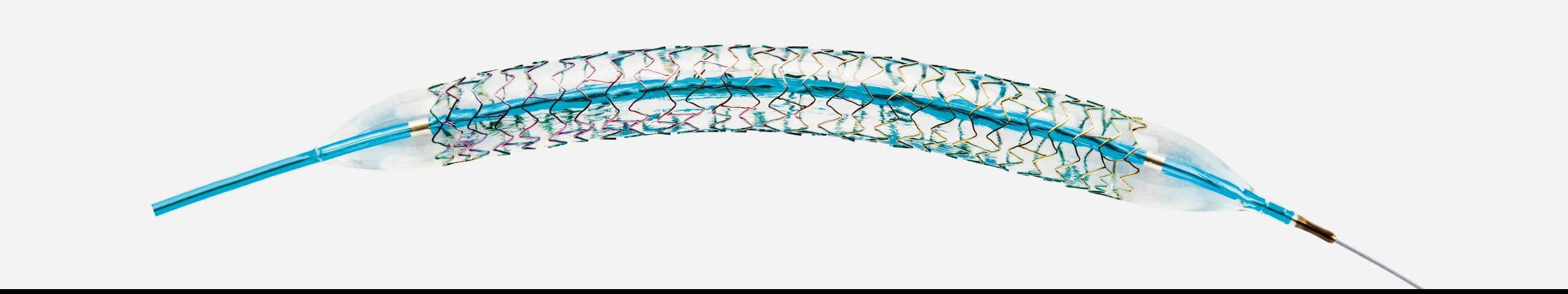
Pro-Kinetic Energy
Cobalt Chromium Coronary Stent System
![]() Catalog donwload Pro-Kinetic Energy
Catalog donwload Pro-Kinetic Energy
Indicated for newly formed (de-novo) discrete stenotic lesions and in-stent restenotic lesions. The combined power of stent design, delivery system and proBIO coating at your fingertips.
Key facts:
- 60 μm thin spacers – demonstrably better clinical results
- Highly flexible double spiral stent design for optimal deliverability and adaptability to the vessel
- proBIO surface layer for improved biocompatibility
- The proven design of the Orsiro / PK Papyrus stent provides exceptional deliverability
Pro-Kinetic Energy
Cobalt Chromium Coronary Stent System
![]() Catalog donwload Pro-Kinetic Energy
Catalog donwload Pro-Kinetic Energy
Indicated for newly formed (de-novo) discrete stenotic lesions and in-stent restenotic lesions. The combined power of stent design, delivery system and proBIO coating at your fingertips.
Key facts:
- 60 μm thin spacers – demonstrably better clinical results
- Highly flexible double spiral stent design for optimal deliverability and adaptability to the vessel
- proBIO surface layer for improved biocompatibility
- The proven design of the Orsiro / PK Papyrus stent provides exceptional deliverability

Clinically proven results

Ultrathin struts

Exceptional deliverability

Clinical proven results

Ultrathin struts

Exceptional deliverability
Product details
Improved stent design
The PRO-Kinetic Energy stent design provides exceptional flexibility without compromising reinforcement or fatigue resistance. This improved stent design provides a smooth outer bend relief. The helical folds provide stent flexibility for excellent deliverability and a smooth profile. Wedge transitions at the end of the stent ensure consistent reinforcement throughout the length of the stent.
Longitudinal connectors provide stability for optimal reinforcement and support without compromising flexibility.
Durable materials allow you to push the boundaries of design with a new concept for thinner struts without affecting other stent properties.
proBIO layer of silicon carbide
The proBIO layer serves as a diffusion barrier, sealing the metal surface and limiting the release of ions. In vitro studies have shown a reduction in allergic metal ions of up to 96% when the surface of the stent is covered with a layer of silicon carbide. Thanks to the barrier against ion release, the silicon carbide layer creates a surface that reduces platelet aggregation and at the same time aids in endothelialization.
Product details
Improved stent design
The PRO-Kinetic Energy stent design provides exceptional flexibility without compromising reinforcement or fatigue resistance. This improved stent design provides a smooth outer bend relief. The helical folds provide stent flexibility for excellent deliverability and a smooth profile. Wedge transitions at the end of the stent ensure consistent reinforcement throughout the length of the stent.
Longitudinal connectors provide stability for optimal reinforcement and support without compromising flexibility.
Durable materials allow you to push the boundaries of design with a new concept for thinner struts without affecting other stent properties.
proBIO layer of silicon carbide
The proBIO layer serves as a diffusion barrier, sealing the metal surface and limiting the release of ions. In vitro studies have shown a reduction in allergic metal ions of up to 96% when the surface of the stent is covered with a layer of silicon carbide. Thanks to the barrier against ion release, the silicon carbide layer creates a surface that reduces platelet aggregation and at the same time aids in endothelialization.
Technical data
| Stent | |
|---|---|
| Stent material | Cobalt chromium, L-605 |
| Passive coating | proBIO (Amorphous Silicon Carbide) |
| Strut thickness | ø 2.0 – 3.0 mm: 60 μm (0.0024″); |
| ø 3.5 – 4.0 mm: 80 μm (0.0031″); | |
| ø 4.5 – 5.0 mm: 120 μm (0.0047″); | |
| Delivery System | |
|---|---|
| Catheter type | Rapid exchange |
| Recommended guide catheter | 5F (min. I.D. 0.056″) |
| Lesion entry profile | 0.017″ |
| Guide wire diameter | 0.014″ |
| USable catheter length | 140 cm |
| Ballon material | Semi-crystalline Co-Polymer material |
| Coating (distal shaft) | Hydrophilic coated |
| Markers band | Two swaged platinum-iridium markers |
| Proximal shaft diameter | 2.0F |
| Distal shaft diameter | 2.5F: ø 2.0 – 3.5 mm; 2.8F: ø 4.0 – 5.0 mm |
| Nominal pressure (NP) | 9 atm |
| Rated burst pressure (RBP) | 16 atm (2.0 – 4.0 mm); 14 atm (4.5 – 5.0 mm) |
Technical data
| Stent | |
|---|---|
| Stent material | Cobalt chromium, L-605 |
| Passive coating | proBIO (Amorphous Silicon Carbide) |
| Strut thickness | ø 2.0 – 3.0 mm: 60 μm (0.0024″); |
| ø 3.5 – 4.0 mm: 80 μm (0.0031″); | |
| ø 4.5 – 5.0 mm: 120 μm (0.0047″); | |
| Delivery System | |
|---|---|
| Catheter type | Rapid exchange |
| Recommended guide catheter | 5F (min. I.D. 0.056″) |
| Lesion entry profile | 0.017″ |
| Guide wire diameter | 0.014″ |
| USable catheter length | 140 cm |
| Ballon material | Semi-crystalline Co-Polymer material |
| Coating (distal shaft) | Hydrophilic coated |
| Markers band | Two swaged platinum-iridium markers |
| Proximal shaft diameter | 2.0F |
| Distal shaft diameter | 2.5F: ø 2.0 – 3.5 mm; 2.8F: ø 4.0 – 5.0 mm |
| Nominal pressure (NP) | 9 atm |
| Rated burst pressure (RBP) | 16 atm (2.0 – 4.0 mm); 14 atm (4.5 – 5.0 mm) |
Compliance Chart
| | Balloon Diameter x Length (mm) | |||||||||
| ø 2.0 | ø 2.25 | ø 2.5 | ø 2.75 | ø 3.0 | ø 3.5 | ø 4.0 | ø 4.5 | ø 5.0 | ||
| x | x | x | x | x | x | x | x | x | ||
| 9-20 | 9-20 | 9-22 | 9-30 | 9-30 | 9-40 | 9-40 | 13-40 | 13-40 | ||
| Nominal Pressure | atm | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
| (NP) | ø (mm) | 2.0 | 2.25 | 2.5 | 2.75 | 3.0 | 3.50 | 4 | 4.5 | 5.0 |
| Rated Burst Pressure | atm | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 14 | 14 |
| (RBP) | ø (mm) | 2.33 | 2.59 | 2.83 | 3.12 | 3.42 | 4.07 | 4.65 | 5.11 | 5.63 |
Compliance Chart
| | Balloon Diameter x Length (mm) | |||||||||
| ø 2.0 | ø 2.25 | ø 2.5 | ø 2.75 | ø 3.0 | ø 3.5 | ø 4.0 | ø 4.5 | ø 5.0 | ||
| x | x | x | x | x | x | x | x | x | ||
| 9-20 | 9-20 | 9-22 | 9-30 | 9-30 | 9-40 | 9-40 | 13-40 | 13-40 | ||
| Nominal Pressure | atm | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
| (NP) | ø (mm) | 2.0 | 2.25 | 2.5 | 2.75 | 3.0 | 3.50 | 4 | 4.5 | 5.0 |
| Rated Burst Pressure | atm | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 14 | 14 |
| (RBP) | ø (mm) | 2.33 | 2.59 | 2.83 | 3.12 | 3.42 | 4.07 | 4.65 | 5.11 | 5.63 |